Antifungal activity of some essential oils against the post-harvest fungal pathogens of guava (Psidium guajava L.)
DOI:
https://doi.org/10.71336/jabs.1442Keywords:
Aromatic Plant, Chemical Fungicides, Fungitoxicity, Growth rate, Medicinal ValueAbstract
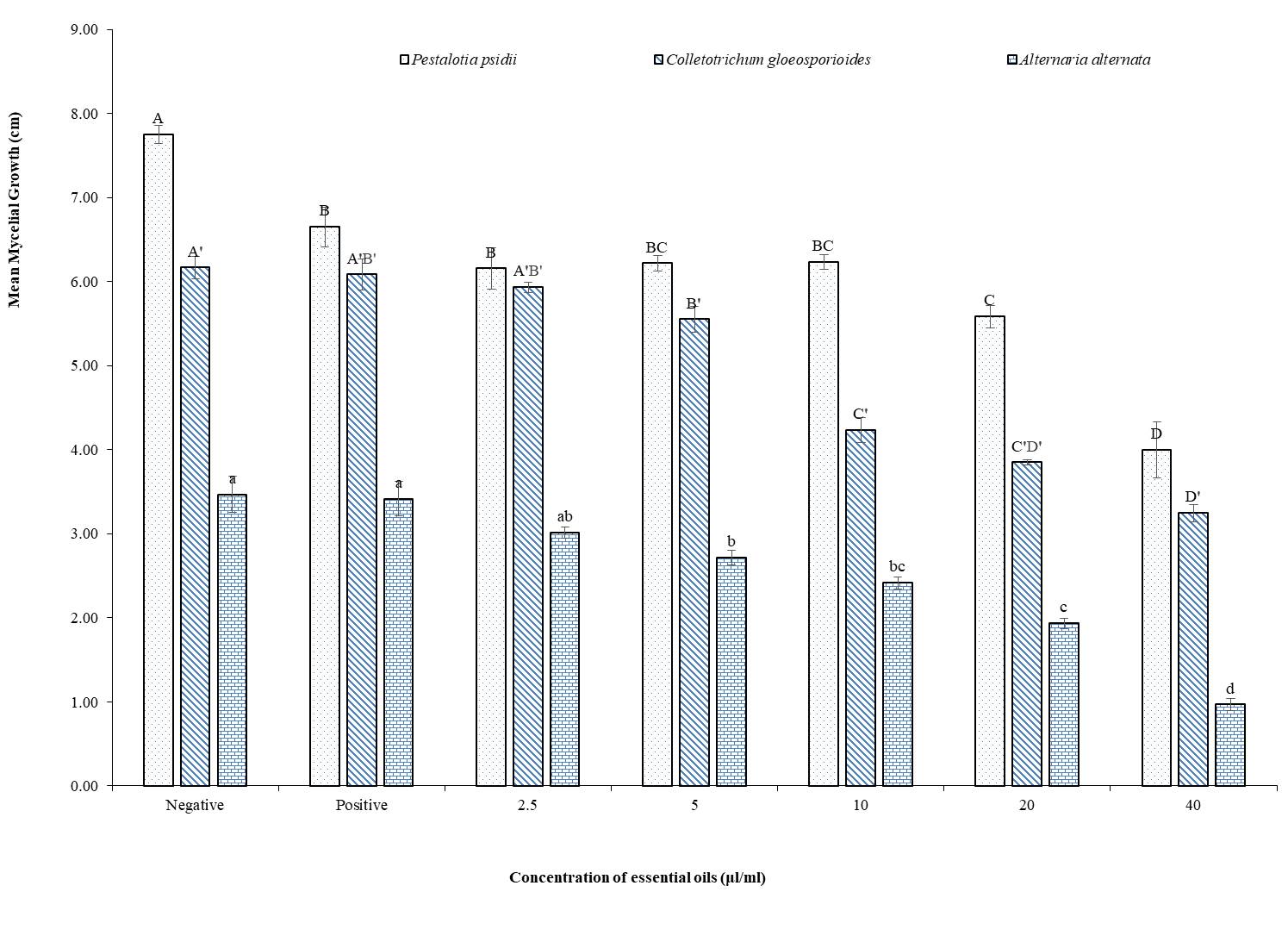
Guava (Psidium guajava L.) is a nutritionally and medicinally valuable fruit, but postharvest losses are primarily caused by fungal pathogens at various stages of harvesting. Chemical fungicides, while effective, pose risks to human health and the environment, prompting the search for natural alternatives. Essential oils from aromatic plants have demonstrated significant antifungal properties, making them potential substitutes for synthetic fungicides. This study evaluated the antifungal efficacy of essential oils against postharvest fungal pathogens isolated from guava fruit. A total of eight fungal species were identified: Alternaria alternata, Aspergillus flavus, Aspergillus niger, Aspergillus versicolor, Colletotrichum gloeosporioides, Monilia fruticola, Penicillium sp, and Pestalotia psidii. Essential oils from Acorus calamus, Callistemon citrinus, and Juniperus indica were tested against A. alternata, C. gloeosporioides, and P. psidii. Essential oils were extracted through hydrodistillation using Cleavinger’s apparatus, followed by GC-MS analysis to characterize their physicochemical properties relevant to antifungal activity. All tested oils significantly inhibited mycelial growth, with A. calamus exhibiting complete inhibition at 20 μl/ml and 40 μl/ml concentrations. These findings highlight the potential of A. calamus essential oil as a natural fungicide to control postharvest fungal pathogens. However, further in vivo research is necessary to assess its efficacy under storage conditions, along with evaluations of toxicity, sensory impact, and economic feasibility for commercial application.
References
[1] Porat, R., Lichter, A., Terry, L. A., Harker, R., Buzby, J. (2018): Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biology and Technology 139: 135–149. https://doi.org/10.1016/j.postharvbio.2017.11.019
[2] Droby, S. (2006): Improving quality and safety of fresh fruits and vegetables after harvest by the use of biocontrol agents and natural materials. Acta Horticulturae 709: 45–52. https://doi.org/10.17660/ActaHortic.2006.709.5
[3] Kumar, A., Singh, J. P., Singh, P., Javeria, S. (2021): Postharvest Diseases of Papaya and Their Management. In: Singh, D., Sharma, R.R., Devappa, V., & Kamil, D. (Eds.) Postharvest Handling and Diseases of Horticultural Produce, CRC Press, Boca Raton, Florida, USA, pp. 239-248. https://doi.org/10.1201/9781003045502-20
[4] Shrestha, A. K. (2005): Critical appraisal of management practices in Nepalese guava orchards. Journal of the Institute of Agriculture and Animal Science 26: 127-133. https://doi.org/10.3126/jiaas.v26i0.665
[5] Fatima, S. (2019): Introduction to major post-harvest diseases of guava. Journal of Drug Delivery and Therapeutics 9: 591–593. https://doi.org/10.22270/jddt.v9i4.3592
[6] Liu, H., Wei, S., Shi, L., Tan, H. (2023): Preparation, structural characterization, and bioactivities of polysaccharides from Psidium guajava: A review. Food Chemistry 411: 135-423. https://doi.org/10.1016/j.foodchem.2023.135423
[7] Sharma, R. R., Singh, D., Singh, R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biological Control 50: 205–221. https://doi.org/10.1016/j.biocontrol.2009.05.001
[8] Weisenburger, D. D. (1993): Human health effects of agricultural use. Human Pathology 24: 571-576. https://doi.org/10.1016/0046-8177(93)90234-8
[9] Pitarokili, D., Tzakou, O., Couladis, M., Verykokidou, E. (1999): Composition and Antifungal Activity of the Essential Oil of Salvia pomiferasub sp. calycina Growing Wild in Greece. Journal of Essential Oil Research 11: 655–659. https://doi.org/10.1080/10412905.1999.9701233
[10] Adhikari, H. S., Jha, S. K. (2023): Postharvest mycobial contaminants of white button mushroom (Agaricus bisporus) and their management using plant essential oils. International Journal of Agriculture Environment and Food Sciences 7(3): 500-507. https://doi.org/10.31015/jaefs.2023.3.4
[11] Barnett, H. L., Hunter, B. B. (1972): Illustrated genera of imperfect fungi. Burgess Publishing Co., Broken Arrow, USA. Pp. 1-185. https://www.academia.edu/31570962/Book_Illustrated_Genera_of_Imperfect_Fungi
[12] Watanabe, T. (2010): Pictorial atlas of soil and seed fungi: morphology of cultured fungi and key to species, CRC press, Boca Raton, Florida, USA. https://archive.org/details/pictorialatlasof0000wata
[13] Rao, G. P. and Srivastava, A. K. (1994): Toxicity of essential oils of higher plants against fungal pathogens of sugarcane. Current Trend in Sugarcane Pathology 15(2): 347-365
[14] Grove, R. K., Moore, J. D. (1962): Toximetric studies of fungicides against brown rot organism Sclerotina fruticola. Phytopathology 52: 867-880. https://www.cabidigitallibrary.org/doi/full/10.5555/19631101156
[15] Vincent, J. M. (1947). Distortion of Fungal Hypha in the Presence of Certain Inhibitors. Nature, 159: 850–850. https://doi.org/10.1038/159850b0
[16] Lal, B., Rai, R.N., Arya, A., Tewari, D.K. (1980). A new rot of Psidium guajava L. National Academy of Science Letters 3: 361-362. https://www.cabidigitallibrary.org/doi/full/10.5555/19821381187
[17] Nongmaithem, N. (2014): Control of post-harvest fungal diseases of guava by essential oil of Azadirachta indica. Indian Journal of Hill Farming 2(1): 238-246. https://epubs.icar.org.in/index.php/IJHF/article/view/46890
[18] Sharma, M. (2021). Postharvest Diseases of Guava (Psidium guajava L.) and Their Management. In: Singh, D., Sharma, R.R., Devappa, V., & Kamil, D. (Eds.) Postharvest Handling and Diseases of Horticultural Produce, CRC Press, Boca Raton, Florida, USA, pp. 219-230. https://doi.org/10.1201/9781003045502
[19] Kaushik, C. D., Thakur, D. P., Chand, J. N. (1972): Parasitism and control of Pestalotia psidii causing cankerous disease of ripe guava fruits. Indian Phytopathology 25: 61-64. https://www.cabidigitallibrary.org/doi/full/10.5555/19731303412
[20] Misra, A.K. (2006). Wilt of guava - a disease of national importance. Indian Phytopathology 59: 269-280. https://epubs.icar.org.in/index.php/IPPJ/article/view/17362
[21] Utikar, P. G., Shinde, P. A., Soawane, C. S. (1986): Influence of temperature and incubation period on fruit for initiation and development by postharvest fungi of guava. Current Research Reporter, Mahatma Phule Agricultural University 2: 209–211. https://www.cabidigitallibrary.org/doi/full/10.5555/19891129407
[22] Adams, R. P. (2011): Junipers of the World: The Genus Juniperus. Trafford Publishing, Victoria BC, Canada.
[23] Zandi-Sohani, N., Hojjati, M., Carbonell-Barrachina, A. A. (2012): Volatile Composition of the Essential Oil of Callistemon citrinus Leaves from Iran. Journal of Essential Oil-Bearing Plants 15: 703–707. https://doi.org/10.1080/0972060X.2012.10644109
[24] Bhandari, N. L., Khadka, S., Dhungana, B. R., Bhandari, G., Bhatt, T. D., Pandey, D. P. P. (2021): Study of phyto-and physicochemical analysis, antimicrobial and antioxidant activities of essential oil extract of Callistemon citrinus (curtis) skeels leaves. Advanced Journal of Chemistry-Section B 3(2): 109-119. https://doi.org/10.22034/ajcb.2021.254741.1067
[25] Lal, M., Borah, A., Pandey, S. K. (2019): Identification of a New High Essential Oil Yielding Variety “Jor Lab AC-1” of Acorus calamus L. Journal of Essential Oil Bearing Plants 22: 695–703. https://doi.org/10.1080/0972060x.2019.1653797
[26] Lohani H., Haider, S. Z., Chauhan, N. K., Mohan, M. (2010): Essential oil composition of leaves and berries of Juniperus communis and Juniperus indica from Uttarakhand Himalaya. Journal of Medicinal and Aromatic Plant Sciences 32: 199–201. https://jmaps.in/
[27] Shukla, R., Singh, P., Prakash, B., Dubey, N. K. (2016): Assessment of Essential Oil of Acorus calamus L. and its Major Constituent β-Asarone in Post Harvest Management of Callosobruchus chinensis L. Journal of Essential Oil Bearing Plants 19: 542–552. https://doi.org/10.1080/0972060X.2014.901627
[28] Radusienė, J., Judzentiene, A., Peciulyte, D., Janulis, V. (2007): Essential oil composition and antimicrobial assay of Acorus calamus leaves from different wild populations. Plant Genetic Resources: Characterization and Utilization 5: 37–44. https://doi.org/10.1017/S1479262107390928
[29] Sameza, M. L., Tchameni, S. N., Ekoue, J. D. A., Jazet, P. M. D., Tchoumbougnang, F. (2016): Growth inhibition of the stored fish (Ethmalosa fimbriata) fungus Aspergillus flavus, exposed to extracted essential oils from Callistemon citrinus and Ocimum canum. African Journal of Microbiology Research 10(30): 1164-1172. https://doi.org/10.5897/AJMR2016.8028
[30] Wu, Y.-X., Zhang, Y. D., Li, N., Wu, D. D., Li, Q. M., Chen, Y. Z., Zhang, G. C., Yang, J. (2022): Inhibitory effect and mechanism of action of juniper essential oil on gray mold in cherry tomatoes. Frontiers in Microbiology 13: 1000526. https://doi.org/10.3389/fmicb.2022.1000526
[31] Angioni, A., Barra, A., Russo, M. T., Coroneo, V., Dessí, S., Cabras, P. (2003): Chemical composition of the essential oils of Juniperus from ripe and unripe berries and leaves and their antimicrobial activity. Journal of agricultural and food chemistry 51(10): 3073-3078. https://doi.org/10.1021/jf026203j
[32] Aljaiyash, A. Ghami, M., Satrani, B., Labiad, H., Echchelh, A., Chaouch, A. (2016): Chemical Composition of Essential Oils of Ripe and Unripe Berries and Leaves of Juniperus phoenicea L. and Determination of their Antimicrobial Activities. International journal of Emerging Engineering Research and Technology 4 (10): 7-14. https://ijeert.ijrsset.org/papers/v4-i10/2.pdf
[33] Sintawarak, P., Uthairatsamee, S., Keawgrajang, T. (2020): In Vitro and In Vivo Inhibition of Cylindrocladium reteaudii by Essential Oils of Acorus calamus Rhizomes. Environment and Natural Resources Journal 19: 34–42. https://doi.org/10.32526/ennrj/19/2020076
[34] Yami, H., Shukla. (2016): Antifungal activity of essential oils derived from some plants against phytopathogenic fungi. Annals of Plant Sciences 5: 1374. https://doi.org/10.21746/aps.2016.07.002
[35] Dethoup, T., Songkumarn, P., Sirirak, T., Kijjoa, A. (2019): Fungicidal activity of Acorus calamus L. extracts against plant pathogenic fungi. Agriculture and Natural Resources 53(5): 527-532. https://li01.tci-thaijo.org/index.php/anres/article/view/226217
[36] Oliveira Filho, J. G., Silva, G. da C., Cipriano, L., Gomes, M., Egea, M. B. (2021): Control of postharvest fungal diseases in fruits using external application of RNAi. Journal of Food Science 86: 3341–3348. https://doi.org/10.1111/1750-3841.15816
[37] Adhikari, H. S., Jha, S. K. (2017): Postharvest microbial contamination in oyster mushroom and their management using plant Essential oils. Bio Bulletin 3(1): 104-108. https://www.biobulletin.com/articles/postharvest-microbial-contamination-in-oyster-mushroom-and-their-management-using-plant-essential-oils.pdf
[38] Jobling, J. (2000): Essential oils: A new idea for postharvest disease control. Good fruit and vegetables magazine 11(3): 50-54. https://web.archive.org/web/20180721194224id_/http://www.postharvest.com.au/GFV_oils.PDF
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Journal of Applied Biological Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.

